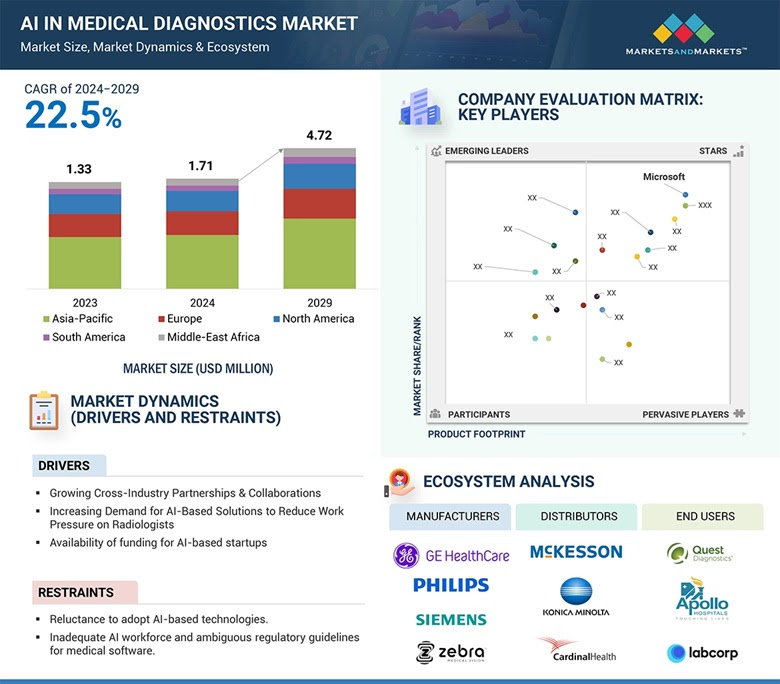
AI in pharma marketing is finally becoming impossible to ignore. The global AI in medical diagnostics market alone is projected to grow from 1.71 billion USD in 2024 to 4.72 billion USD by 2029, a blistering 22.5 percent annual growth. That isn’t a vague forecast; it’s evidence that the adoption of intelligent systems in healthcare is accelerating everywhere. Yet inside most Indian pharma and healthcare organisations, the real obstacle isn’t the technology itself. It’s whether teams, doctors, and compliance heads trust it enough to let it run.
Technology isn’t the missing piece. The missing piece is confidence that the system will work inside a regulated, risk-averse, and reputation-sensitive environment. The real challenge isn’t to “deploy AI.” It’s to make it explainable, auditable, and aligned with how Indian healthcare actually operates. And until marketing, medical affairs, and IT work together on that foundation, the hype around AI in pharma marketing will stay exactly that- hype.
What is AI in pharma marketing
When we speak of AI in pharma marketing, we’re not referring to some futuristic robot doctor. We’re talking about a data-driven ecosystem that learns from patterns across digital, medical, and field interactions and uses those insights to guide communication, targeting, and decision-making.
AI in pharma marketing means systems that analyse vast datasets from multiple touchpoints like CRM entries, e-mail opens, webinar attendance, website activity, field-force logs, and even anonymised diagnostic data; and then convert that noise into action. These systems can predict which doctors are most receptive to a particular therapy message, which content combinations work best for which specialty, and how soon to follow up after a prescription trend shifts.
It is also about automation of compliance workflows, where content is checked against reference libraries and legal language in real time, reducing the endless review cycles that bog down Indian pharma campaigns. Hence, AI in pharma marketing isn’t about replacing marketers or reps. It’s about replacing guesswork with intelligence.
What makes this transition hard is that most pharma companies still operate in silos. Marketing creates content. Medical reviews it. Compliance approves it weeks later. By then, the opportunity is gone. AI can close those gaps only when the organisation is ready to trust machine-assisted decisions and when compliance is built into the workflow from the first draft.
Why adoption lags even when technology exists
If technology were the real issue, the 4.72 billion-dollar diagnostics market wouldn’t exist. The systems work. The models are ready. Yet Indian pharma teams hesitate because trust and governance are still playing catch-up.
The first layer of hesitation comes from clinicians.
Doctors are trained to rely on data they can see and studies they can quote. When an algorithm recommends an action without showing how it reached that conclusion, the natural reaction is scepticism. They are not wrong. In healthcare, wrong decisions cost lives. So when marketing teams push AI-driven segmentation or personalised outreach, clinicians ask a fair question: Can you prove this is accurate, ethical, and compliant?
The second layer is internal.
Many marketing and medical teams simply don’t trust what they can’t control. If an AI engine reorders campaign priorities or suggests content changes, teams fear it might misinterpret clinical nuance or local regulations. In a sector where a single non-compliant claim can trigger regulatory scrutiny, that’s a rational fear.
Then there’s the infrastructure problem.
Legacy CRMs, outdated content systems, and scattered analytics setups mean data is fragmented. The algorithm cannot learn what it cannot see. So even the most advanced AI systems, once plugged in, choke on inconsistent or incomplete data.
Finally, there’s the policy vacuum.
The diagnostic market report highlighted the lack of clear regulatory frameworks for AI-based medical software. The same ambiguity applies to marketing and engagement. Who is accountable if an AI-generated suggestion leads to a misleading message? India doesn’t yet have a concrete answer, and organisations are understandably cautious.
Benefits of AI in pharma marketing
Despite all that, the case for adoption is overwhelming. The benefits are no longer theoretical. Globally, AI is already proving that when done responsibly, it transforms marketing efficiency and medical accuracy simultaneously.
AI in pharma marketing allows precision that traditional segmentation never could.
Instead of blanketing every physician with the same campaign, systems learn behavioural patterns from digital interactions and field feedback to identify which messages resonate with which clusters of doctors. This is not creative optimisation; it is measurable science. The result is higher engagement and better alignment between what the doctor actually needs and what the brand communicates.
Speed is another advantage.
In most Indian companies, launching a new campaign takes weeks because every asset moves through multiple departments and manual approvals. AI-driven content management systems can track, validate, and route materials automatically. The outcome is faster approvals, shorter go-to-market cycles, and reduced dependency on endless email chains.
Most importantly, AI brings visibility.
Marketers can finally connect inputs to outcomes, which means understanding which activities lead to genuine prescription shifts or educational uptake. In an industry obsessed with compliance, this traceability is gold. When you can show an auditable trail from message to outcome, you not only build trust with regulators but also prove the commercial value of compliant marketing.
The human impact shouldn’t be underestimated either. Just as radiologists use AI to lighten diagnostic workloads, marketing teams use it to remove repetitive, low-value tasks. Analysts stop spending nights merging Excel sheets. Reviewers focus on genuinely complex approvals. Reps walk into meetings armed with insights, not guesses.
How AI is used in pharmaceutical marketing
To understand the real-world potential, imagine a pharma organisation that treats its marketing and medical systems as a connected ecosystem. Every engagement, such as a webinar, a patient-education microsite, or an e-detailer interaction, feeds structured data into a central engine. The system analyzes that flow in real time and recommends the next step.
If a cardiologist reads a new clinical summary but ignores the follow-up email, the system flags that behaviour and suggests a different channel, perhaps a WhatsApp alert with an educational video. If a neurologist downloads three case studies about side effects, the system prompts the marketing team to invite her to an upcoming safety webinar. The decision is not random; it’s driven by thousands of behavioural patterns across the network.
On the content side, AI automates the slowest, most expensive part of pharma marketing, the approval loop. Every brand manager knows the pain of waiting weeks for MLR sign-offs. AI can pre-screen content, cross-verify claims, and highlight potential breaches before the file ever reaches human reviewers. Instead of checking every comma, compliance officers focus on the few cases that genuinely need attention.
For analytics teams, AI links engagement data with business outcomes. It becomes possible to correlate digital activity with sales data, understand regional differences in prescription behaviour, and forecast where to invest next. Over time, the system doesn’t just describe what happened; it predicts what to do next.
That is how AI is used in pharmaceutical marketing when it’s implemented correctly, not as a side experiment but as an operational backbone that informs every marketing and medical decision.
Why this must be treated as a boardroom issue
The companies that will lead this decade are the ones that realise AI in pharma marketing is not a marketing project. It’s a boardroom transformation.
When a pharma company invests in AI, it isn’t buying software; it’s changing how it thinks about compliance, agility, and growth. The marketing head, CIO, and medical director must work together because this technology touches everything from data governance to campaign ethics. In most Indian firms, these functions still work in isolation. That’s precisely why projects stall.
Treating AI adoption as an IT initiative is a common mistake. It ends up buried under procurement, timelines, and budget cycles. In reality, this is a strategic move that affects market share. When diagnostic companies worldwide can scale at 22 percent a year using AI to improve accuracy, pharma cannot afford to remain manual in its engagement with doctors and patients.
Building this capability requires a mindset shift. Compliance must be seen not as a barrier but as the foundation. Data integration cannot be postponed; it must precede everything else. Teams need to see AI not as a threat but as a safety net that makes their decisions smarter and their audits cleaner.
How to bridge the trust and compliance gap
There’s no shortcut to trust. It’s earned through transparency, explainability, and consistent delivery. For AI to gain acceptance in pharma, systems must be auditable from the first line of code. Every decision an algorithm makes, whether it’s ranking doctors or approving content, should leave a visible trail that humans can verify. When marketers and compliance officers can see why a recommendation was made, they begin to trust the process.
Embedding compliance inside workflows is equally important. Too many companies treat regulatory checks as the final stage, after the campaign is ready. By then, delays are inevitable. When compliance rules are coded into the system, potential breaches are flagged in real time. This doesn’t just prevent violations; it builds confidence that technology is an ally, not a liability.
The smartest companies also pilot their AI initiatives. They start with one therapy area, one region, or one content process. They measure the difference between traditional and AI-assisted execution, including faster approvals, fewer errors, higher engagement, and use that data to convince internal sceptics. Small wins build organisational belief faster than any presentation can.
Clean, integrated data remains the foundation. Without it, AI becomes a very expensive illusion. Integrating CRM, CMS, and analytics systems might sound like plumbing work, but it’s where most projects either succeed or fail. Once data is unified, models can finally see the full picture, the same way radiology AI systems improve when they access more annotated scans.
Governance must be continuous. Models drift. Regulations evolve. Teams change. A system that isn’t reviewed or retrained periodically will fail quietly. Regular audits, retraining cycles, and bias checks are not optional; they are what keep AI compliant and credible.
And finally, involve clinicians. If doctors don’t trust the outputs, adoption collapses. Feedback loops, advisory sessions, and transparent sharing of algorithm logic make doctors part of the journey. When they see that AI improves patient outcomes or reduces workload, resistance turns into advocacy.
The mindset shift from tools to systems
The real transformation begins when leadership stops thinking of AI as a tool and starts treating it as an evolving system. Tools are installed once and forgotten. Systems are designed, monitored, and continuously improved.
This means the organisation must accept that the technology, compliance, and marketing teams are now one ecosystem. Decisions about data quality, user interfaces, or automation rules are not IT decisions; they are marketing performance decisions. Similarly, compliance frameworks are no longer checklists; they are living guardrails coded into the workflow.
In this environment, success is measured by adoption and impact. Are brand teams using the insights? Are compliance escalations decreasing? Are campaigns going live faster without errors? Those are the new KPIs.
Pharma companies that evolve this way see results quickly. Campaign cycle times shrink. Engagement metrics improve. Compliance queries drop. The technology becomes invisible because it simply works. That’s when AI in pharma marketing stops being a headline and starts being a habit.
The risks if you ignore it
Refusing to evolve is not neutrality; it’s decline. As AI reshapes diagnostics, radiology, and clinical operations globally, marketing will be next. Companies that ignore it will find themselves out-paced by competitors who can reach doctors faster, personalise messages better, and prove ROI more transparently.
The risks of half-hearted adoption are equally serious. Deploying AI without proper governance or explainability can backfire. Models that produce inconsistent results erode credibility. A single compliance error caused by opaque automation can damage both brand and reputation. That’s why a controlled, transparent approach is the only sustainable path.
The results when you get it right
When trust, compliance, and execution align, the outcomes are measurable. Campaign costs drop because fewer iterations are needed. Approvals move faster because reviewers deal only with genuine exceptions. Engagement rates climb because doctors receive information that actually matters to them. Data from different departments finally connects, giving leadership a single view of marketing performance.
The ROI speaks for itself. Time-to-market reduces. Field teams get smarter leads. Digital teams spend less time fixing data and more time creating strategy. Compliance teams become proactive instead of reactive. The organisation stops fearing audits and starts using them as validation of its discipline.
The macro trend supports this direction. With diagnostic AI growing at 22 percent annually, the expectation is already set: healthcare must be smarter, faster, and more precise. For Indian pharma marketers, the question is no longer whether to use AI, but how quickly they can integrate it without compromising trust.
Conclusion
The truth is simple. The gap in AI adoption in Indian healthcare and pharma is not a technology gap. It is a trust and compliance gap. The systems exist. The data is there. The models can be built in weeks. But without transparent design, embedded compliance, and disciplined execution, none of it sticks.
Pharma leaders who understand this will move first. They will treat AI as an enterprise transformation, not a campaign add-on. They will insist on explainable algorithms, integrated data, and workflows where compliance is built in, not bolted on. They will train their teams, run pilots, measure results, and scale confidently.
The others will wait for perfect regulations, perfect talent, and perfect timing, and watch the market move on without them.
Technology has already proven its value. The next step is leadership. And the companies that make that leap now will define what intelligent, compliant, and outcome-driven marketing looks like for the next decade.





