Introduction: Why UCPMP 2024 Is a Turning Point
UCPMP 2024 is not another guideline update. It is a reset for how pharma marketing works in India. For years, many companies have navigated compliance by adding more manual checks, more paperwork, and longer approval cycles. That approach is no longer sustainable. Compliance is non-negotiable in pharma marketing, but neither is speed. Every day lost in Medical-Legal-Regulatory (MLR) approval is a lost day of brand visibility.
Indian pharma CMOs and marketing heads now find themselves in a dual pressure zone. On one side, UCPMP 2024 guidelines demand stricter oversight and accountability. On the other, leadership teams want faster campaigns, sharper digital presence, and measurable HCP engagement. The truth is simple: you cannot meet both demands with yesterday’s processes.
This playbook breaks down UCPMP 2024 into what matters for marketing leaders, why compliance and execution are two sides of the same coin, and how technology-driven workflows are becoming the only way to survive and win. Along the way, you will see why the conversation has shifted from “compliance as cost” to “compliance as advantage.”
Here are the UCPMP Guidelines 2024 that you must read before proceeding further.
Section 1: Understanding UCPMP 2024 in Context
1.1 What UCPMP 2024 Actually Demands
The UCPMP 2024 guidelines extend beyond earlier codes by tightening expectations around promotional practices, doctor engagement, and transparency. The intent is clear: eliminate indirect incentives and force marketing to stand on ethical ground. For marketing leaders, this means approvals will be scrutinized, documentation must be airtight, and every HCP-facing material has to be audit-ready.
For those used to rushing campaigns through MLR, this will feel like a roadblock. But it is not optional. Fines and reputational damage are real risks. If your teams are still managing compliance in spreadsheets and email chains, UCPMP 2024 will expose every weakness.
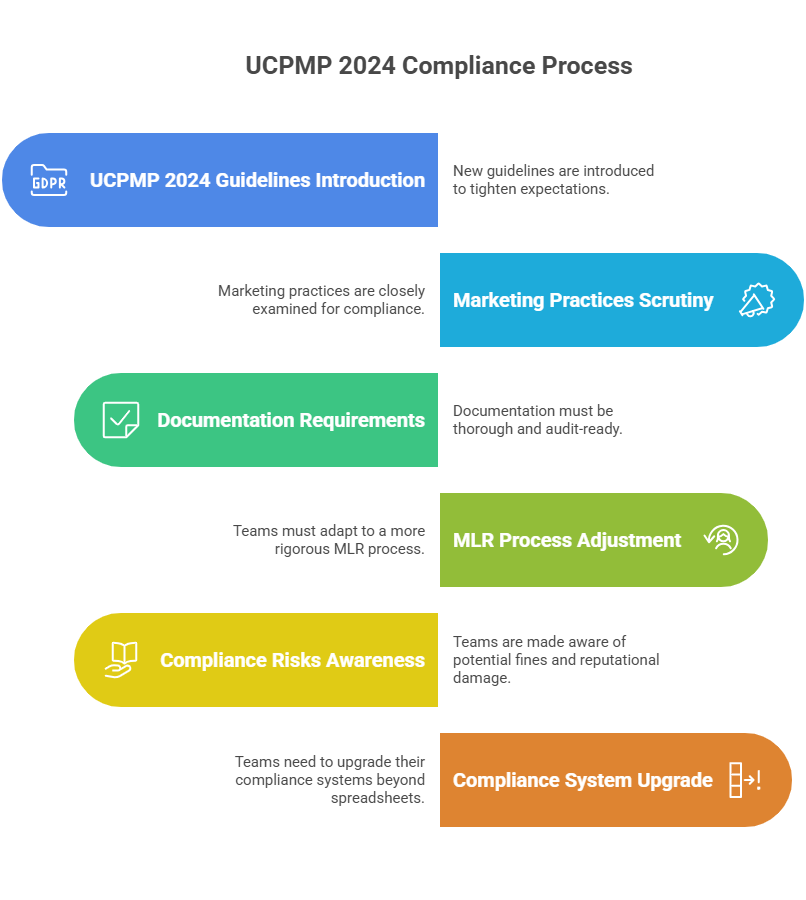
1.2 Why Compliance is Now Strategic, Not Operational
Until now, compliance has been treated as a back-office function. Marketing creates, legal reviews, medical checks boxes, and only then does content go out. That mindset breaks under UCPMP 2024. Compliance is not an afterthought, but defines how marketing must operate.
Forward-looking companies are already reframing compliance as a strategic advantage. If your approval cycles are faster, if your audit trail is cleaner, if your risk of recall is lower, you gain speed and credibility. In a market where doctors are bombarded with generic content, credibility is currency.
1.3 The Hidden Cost of Non-Compliance
Ignoring compliance is not just about penalties. It drags down marketing ROI in less visible ways. Campaign delays mean your competitor reaches HCPs first. Fragmented documentation means rework and duplicate reviews. Compliance bottlenecks create frustrated brand teams who stop pushing digital innovation. By the time your campaign is live, the moment is gone.
This is why pharma marketing compliance in India is no longer just about avoiding risk; it is about enabling execution at the pace your market demands.
Section 2: The MLR Bottleneck and Its Business Impact
2.1 Why Approval Cycles Are Broken
In most Indian pharma companies, MLR approval is still linear. A PowerPoint or PDF is passed along email chains. Every department adds comments in different versions. Consolidation takes weeks. Final approval is given when deadlines are already missed.
This system was never built for omnichannel marketing. It cannot keep up with the demands of digital-first campaigns, where a single initiative may involve email, WhatsApp, portals, webinars, and HCP apps.
2.2 The Pressure from UCPMP 2024
The new guidelines make slow processes even slower if nothing changes. Stricter review means more back-and-forth. Legal and medical teams now want more evidence, more disclaimers, and more proof. For marketers, this is a double hit: longer approvals and stricter scrutiny.
The irony is clear. The more you stick to manual processes, the less compliant you actually are. Every missed disclaimer, every undocumented approval step, every untracked change is a compliance gap waiting to be exposed.
2.3 What It Costs in Real Numbers
Every delay translates to missed revenue. If a brand launch is pushed back by two months due to MLR approval pharma delays, that is two months of zero prescriptions. If a campaign is delayed by weeks, your competitor fills the void.
Brand managers know this pain. They are under pressure to deliver campaigns, but stuck waiting for redlines. CMOs feel it in the boardroom when marketing is asked to prove ROI but cannot show impact because campaigns never reached scale in time.
Here are the Practical execution tactics for shrinking MLR cycles
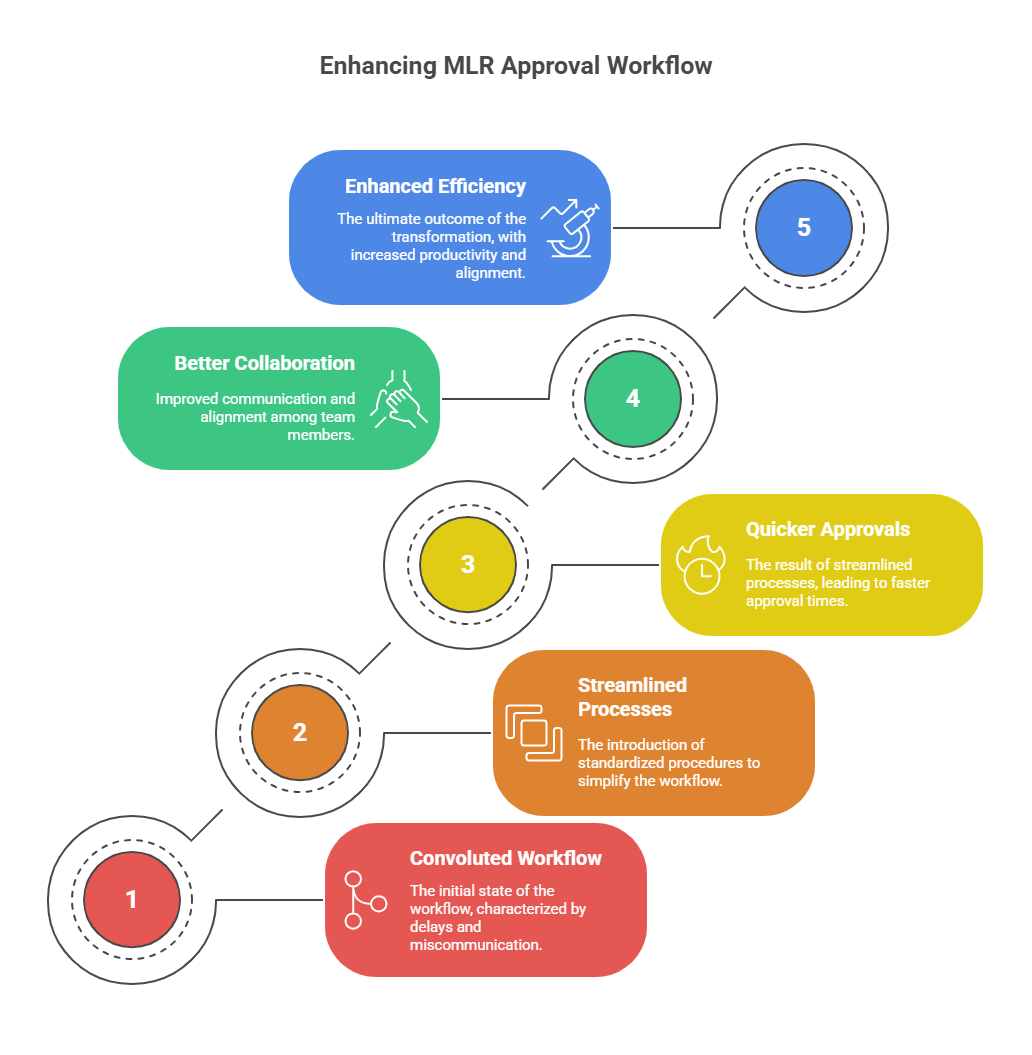
Section 3: Building Compliance Into the System
3.1 Moving from Manual to Modular
The only sustainable fix is moving from document-based workflows to modular content systems. Instead of creating entire campaigns from scratch and sending them for full review, companies are now building libraries of pre-approved content blocks, including claims, graphs, disclaimers, and templates.
When most of the content is already approved, MLR review becomes faster and lighter. Instead of weeks, final checks can take days. This is not theory. Global leaders are already operating this way, and Indian pharma is catching up fast.
See how Valuebound approaches modular content workflows.
3.2 Compliance as Code, Not Paperwork
The mindset shift is simple but powerful: compliance should not live in PDFs. It should be coded into your systems. Modern content engines allow you to embed mandatory disclaimers, enforce approval rules, and maintain audit trails automatically.
Instead of hoping someone remembers to add a line of safety information, the system ensures it cannot be skipped. Instead of relying on emails for proof of review, every action is logged and time-stamped. This is how compliance becomes reliable without being slow.
3.3 Training Teams to Work Differently
No technology will work if teams are not trained. Shifting from ad-hoc content creation to modular workflows requires new habits. Brand teams must think in blocks, not slides. Medical teams must learn to trust system-enforced compliance.
Companies that invest in training upfront see the payoff quickly. Approval times shrink. Frustration drops. Everyone spends more time on strategy and less on chasing redlines.
Section 4: UCPMP 2024 as a Catalyst for Omnichannel
4.1 Why Omnichannel Needs Compliance First
You cannot run omnichannel campaigns without compliance sorted. Every new channel multiplies the risk of errors. An email may require one disclaimer, a WhatsApp message another, and a portal a third. If each asset goes through the old approval cycle, omnichannel is dead on arrival.
4.2 Linking Data, Compliance, and Engagement
The most progressive pharma marketers are building platforms where compliance and engagement sit together. When HCP touchpoints like emails, webinars, and portal visits, are unified in one timeline, compliance oversight is natural. You know exactly what was sent, approved, and when.
This is where our products like Journey (Unified HCP Mapper) and Velocity align. One gives you visibility, the other ensures speed under compliance. Together, they make omnichannel actually possible under UCPMP 2024.
4.3 Lessons from Early Movers
Companies that embraced modular content and unified platforms before 2024 are now ahead. Their omnichannel pilots scaled because approvals did not kill them. Their brand teams are delivering consistent, compliant experiences across channels. Their CMOs can stand in the boardroom and prove ROI with confidence.
This is the new baseline. Everyone else will either catch up or lose ground.
Section 5: From Compliance Burden to Compliance Advantage
5.1 Winning Trust with Faster, Cleaner Approvals
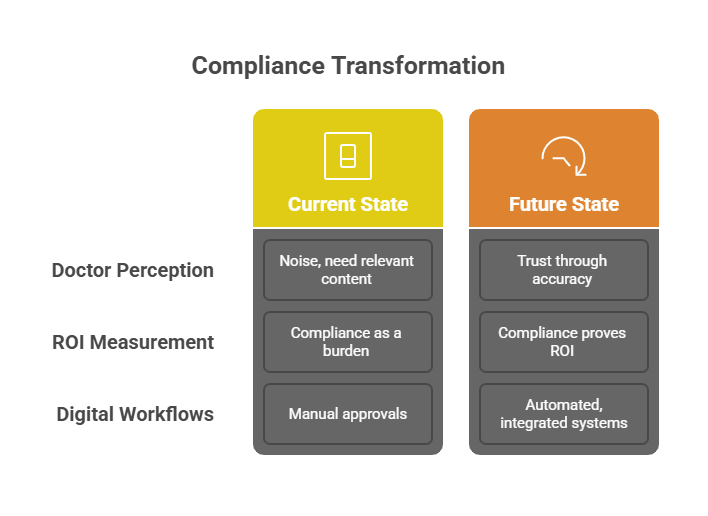
Doctors do not want more noise. They want relevant, credible content. If your approval system ensures accuracy and compliance, your brand gains trust. UCPMP 2024 makes compliance table stakes. Companies that master it will stand out as trustworthy partners for HCPs.
5.2 Using Compliance to Prove ROI
When every approval step is logged, every asset tracked, and every campaign tied to outcomes, compliance becomes proof of ROI. CMOs can show the board not just that campaigns were compliant, but also that they drove measurable engagement.
5.3 What the Future Looks Like
In the next two years, compliance will be fully embedded in digital workflows. Manual approvals will vanish. Modular content, automated audit trails, and integrated analytics will become standard. Companies that move early will not just survive UCPMP 2024. They will use it as a lever for faster, smarter marketing.
Case Study: Cutting Approval Time from 12 Weeks to 3
One of India’s top five pharma companies faced the same problem every brand team knows: endless MLR cycles. Campaigns were stuck for months. Doctors were disengaged. Competitors were faster to market.
By moving to a modular content engine and embedding compliance rules into the workflow, they reduced average approval time from twelve weeks to three. Campaigns launched on schedule. The compliance team was less stressed. Engagement scores improved by 40 percent within the first quarter.
This is not an isolated success. It is what happens when compliance shifts from being a bottleneck to being a system feature.
Read more case studies of pharma MarTech transformation here.
What to Do Next
Compliance is non-negotiable. Speed is non-negotiable. UCPMP 2024 forces you to solve both at the same time. If your approval cycles are still manual, you already know the risk.
The next step is clear: re-engineer your content workflows for compliance and speed together. Companies that do this will not just pass audits. They will win campaigns, win trust, and win market share.
Explore how Valuebound helps Indian pharma build compliance-ready content engines.
Frequently Asked Questions:
What is UCPMP?
UCPMP is the Uniform Code for Pharmaceutical Marketing Practices in India. It sets ethical rules for pharma companies on how they promote medicines to doctors.
What is the difference between UCPMP 2014 and 2024?
UCPMP 2014 was voluntary, lightly enforced. UCPMP 2024 makes compliance mandatory with stricter oversight, tighter review of promotions, and penalties for violations.
What is the national pharmaceutical policy of India?
India’s pharmaceutical policy aims to ensure affordable access to medicines, promote domestic manufacturing, support R&D, and strengthen the global competitiveness of Indian pharma.
What is the outlook for the Indian pharmaceutical industry in 2024?
In 2024, India’s pharma industry is projected to grow 8-10%, driven by generics, digital adoption, exports, and regulatory changes like UCPMP shaping marketing strategies.
What is the goal of Viksit Bharat 2047?
Viksit Bharat 2047 is India’s vision to become a developed nation by its 100th independence year, with goals spanning healthcare, innovation, sustainability, and inclusive growth.





