UCPMP 2024 Guidelines: What Every Pharma Marketer Needs to Know
The UCPMP 2024 guidelines are not just another regulatory update. They mark a decisive shift in how pharmaceutical marketing in India will be judged, tracked, and held accountable. For years, pharma compliance in India was seen as optional, loosely enforced, or handled only at the end of the process. Those days are gone.
For CMOs, brand directors, and digital transformation leaders, the stakes are clear. Marketing teams can no longer afford to treat compliance as paperwork. They must integrate it into the way they plan, create, and launch campaigns. This article breaks down the essentials: what has changed, what it means for marketing operations, and how to prepare with a practical UCPMP 2024 compliance checklist pharma marketers can use.
Read our complete UCPMP 2024 resource here.
Section 1: What the UCPMP 2024 Guidelines Really Mean
1.1 A Quick Look Back
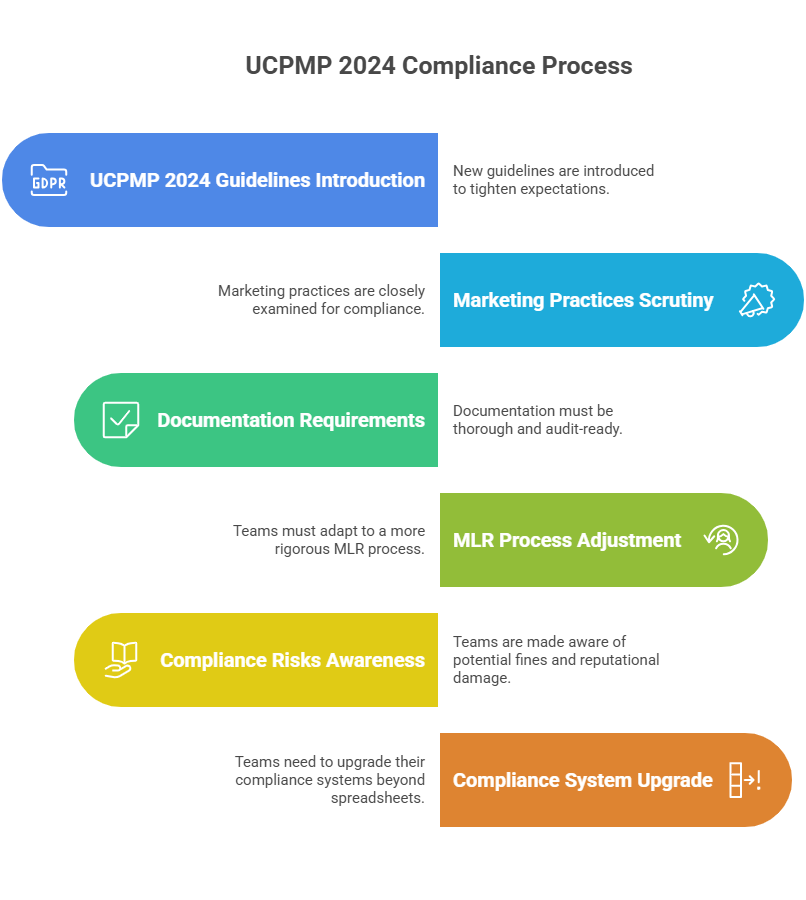
The 2014 version of the Uniform Code for Pharmaceutical Marketing Practices was largely voluntary. Many companies complied in part, but enforcement was patchy. This left room for inconsistent practices like gifting, unstructured doctor interactions, and weak audit trails.
1.2 The 2024 Shift
The UCPMP 2024 guidelines make compliance mandatory. Marketing promotions, HCP engagement, and brand campaigns will be audited. Penalties are more clearly defined. Companies that ignore the rules risk reputational damage and financial cost.
1.3 Why Marketing Cannot Ignore It
These changes directly affect how campaigns are planned and approved. It’s not only a legal matter. Marketing leaders will be held accountable for ensuring every promotional material meets pharma marketing regulations India. This makes compliance a boardroom issue, not an operational afterthought.
Section 2: The Core Principles of Pharma Compliance in India
2.1 Transparency First
The guidelines focus on eliminating hidden incentives. Interactions with HCPs must be transparent. Every event, sponsorship, or content piece should be documented and justified.
2.2 Accuracy of Information
Promotional claims must be backed by evidence. This impacts the way brand teams prepare content. References must be clear, disclaimers visible, and exaggerated claims avoided. Accuracy is not optional; it’s regulated.
2.3 Ethical Promotion
The code sets boundaries for what can and cannot be done in promotion. For example, gifts or lavish hospitality are no longer tolerated. Instead, educational and knowledge-driven engagement is encouraged. For marketers, this means building credibility with content rather than perks.
Section 3: The Practical Challenges Marketing Leaders Face
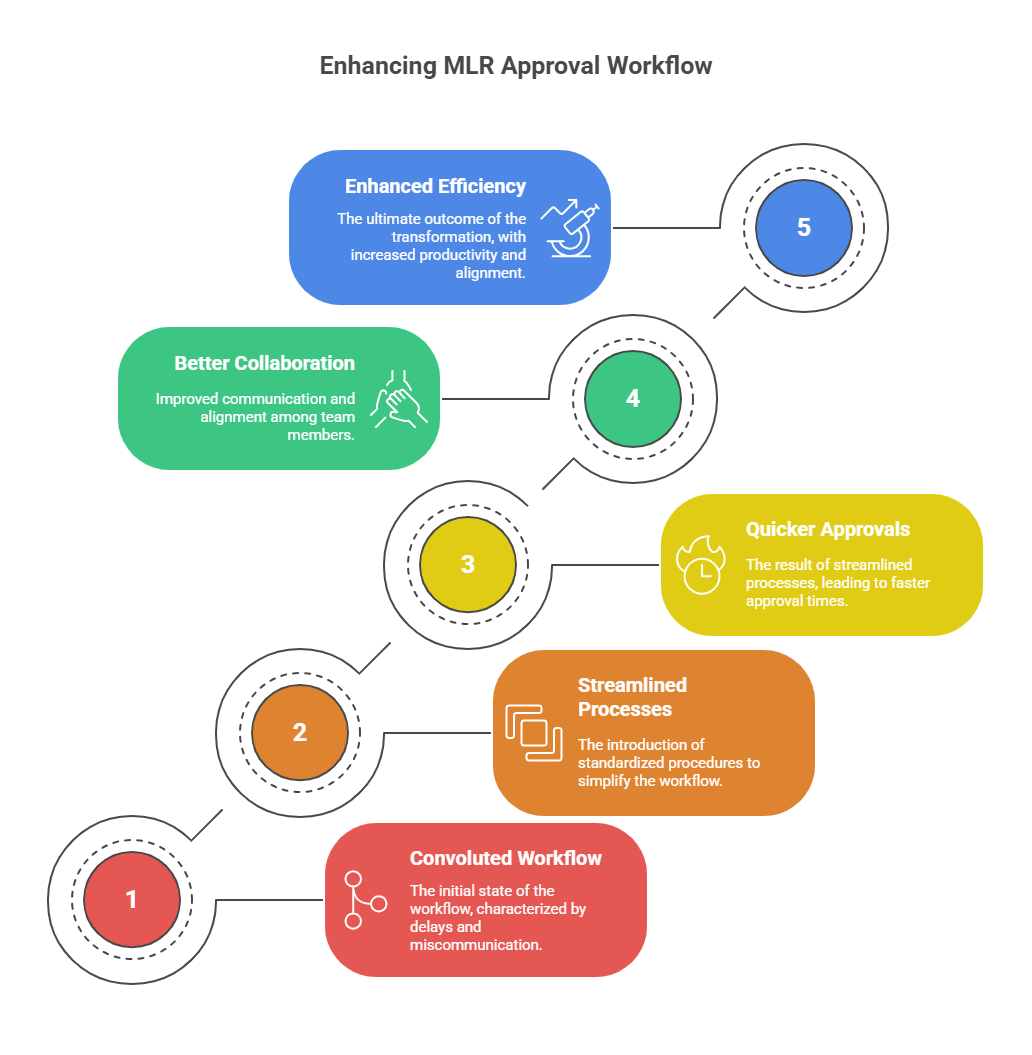
3.1 Approval Cycles Are Slower by Default
With stricter rules, MLR teams are demanding more evidence. Unless workflows are updated, approval cycles will become even slower than before.
3.2 Fragmented Processes Create Risk
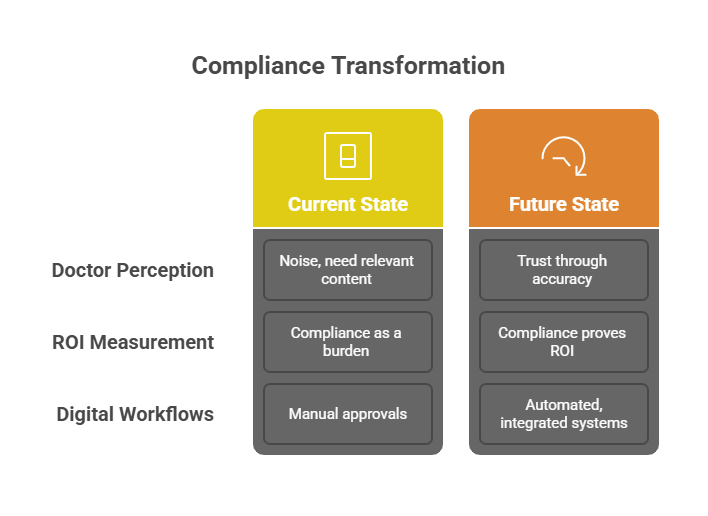
Many companies still rely on spreadsheets, email threads, and scattered repositories for approvals. Under UCPMP 2024, this is dangerous. A missing audit trail could expose the entire campaign to scrutiny.
3.3 The Omnichannel Complexity
Modern pharma marketing is omnichannel: email, WhatsApp, portals, webinars, and sales reps. Every channel multiplies compliance checks. Without integrated workflows, the burden will overwhelm brand teams.
Section 4: Building a UCPMP 2024 Compliance Checklist
4.1 Checklist for Content Creation
- Use only verified claims and references.
- Ensure every asset has mandatory disclaimers.
- Store pre-approved blocks in a central repository.
4.2 Checklist for Approval Workflows
- Define roles for Medical, Legal, and Regulatory review.
- Use systems that track every change and approval.
- Embed compliance rules in the platform, not in email chains.
4.3 Checklist for Post-Campaign Oversight
- Maintain audit trails for every material.
- Track campaign performance with compliance dashboards.
- Schedule periodic reviews to align with new pharma marketing regulations India.
Section 5: Turning Compliance Into Competitive Advantage
5.1 Faster Approvals Mean Faster Launches
Companies that streamline compliance can launch campaigns ahead of competitors. Compliance, when designed well, enables speed.
5.2 Credibility with HCPs
Doctors are more likely to engage with brands they trust. Transparent, accurate, and compliant campaigns earn that trust.
5.3 Proof in the Boardroom
When compliance is embedded in workflows, every decision is documented. CMOs can prove to the board not only that campaigns are ethical, but also that they deliver ROI.
Case Example: From Burden to Advantage
A mid-sized Indian pharma company struggled with fragmented approvals and slow campaigns. After UCPMP 2024, leadership made compliance a strategic priority. They adopted modular content systems, embedded disclaimers, and automated audit trails. Within a year, campaign turnaround time improved by 60%. Audits were cleared with zero red flags. Engagement with doctors improved because content was faster and more credible.
Conclusion: Compliance Is Non-Negotiable, Speed Is Possible
The UCPMP 2024 guidelines raise the bar for pharma compliance in India. They demand accuracy, transparency, and ethical engagement. But they do not have to slow marketing down. With the right workflows, systems, and culture, compliance becomes an enabler, not a blocker.
For CMOs and marketing leaders, the message is clear: adapt now or be left behind. Compliance is not just about risk—it is about building credibility, speed, and long-term advantage in the market.
Frequently Asked Questions:
What are the UCPMP 2024 guidelines?
The UCPMP 2024 guidelines are mandatory rules in India that regulate how pharma companies market medicines, focusing on ethical promotion, accuracy, and transparency.
Is UCPMP compulsory?
Yes. Since 2024, UCPMP has become compulsory for all pharma companies in India. Compliance is enforced, and violations can lead to penalties and reputational damage.
What is UCPMP 2014?
UCPMP 2014 was the earlier version of the pharma marketing code in India. It was voluntary, loosely enforced, and allowed inconsistencies in promotional practices.
Who released UCPMP?
The Department of Pharmaceuticals (DoP), under the Government of India, released UCPMP to regulate marketing practices of pharmaceutical companies nationwide.
What is the purpose of the UCPMP?
The purpose of UCPMP is to ensure ethical, transparent, and compliant marketing of medicines in India, protecting doctors, patients, and the integrity of pharma promotion.